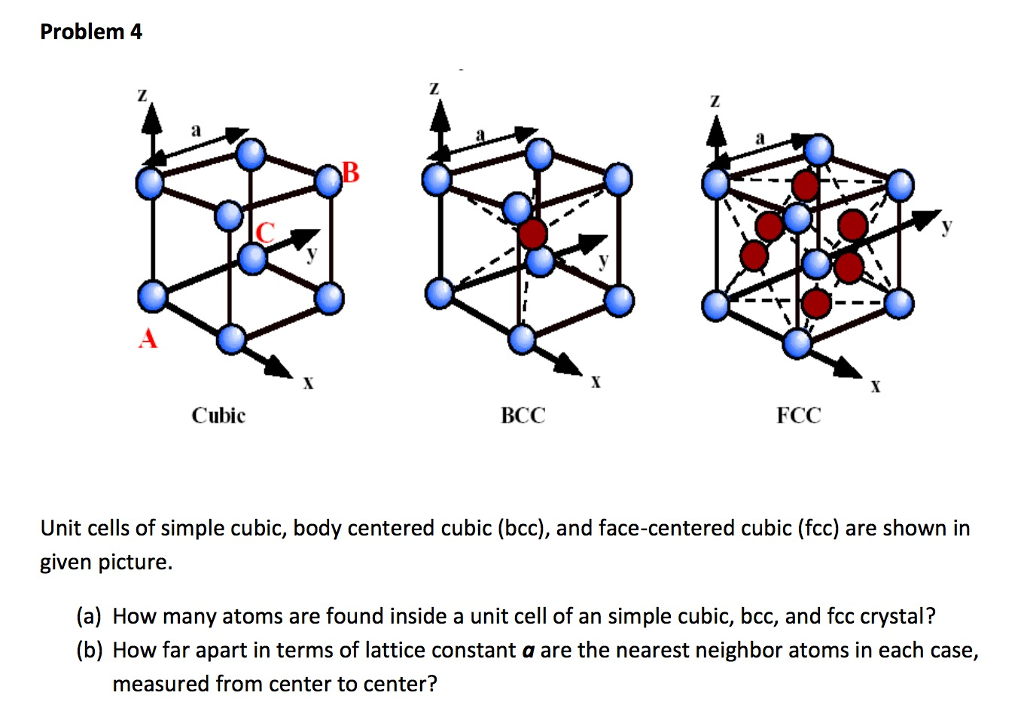
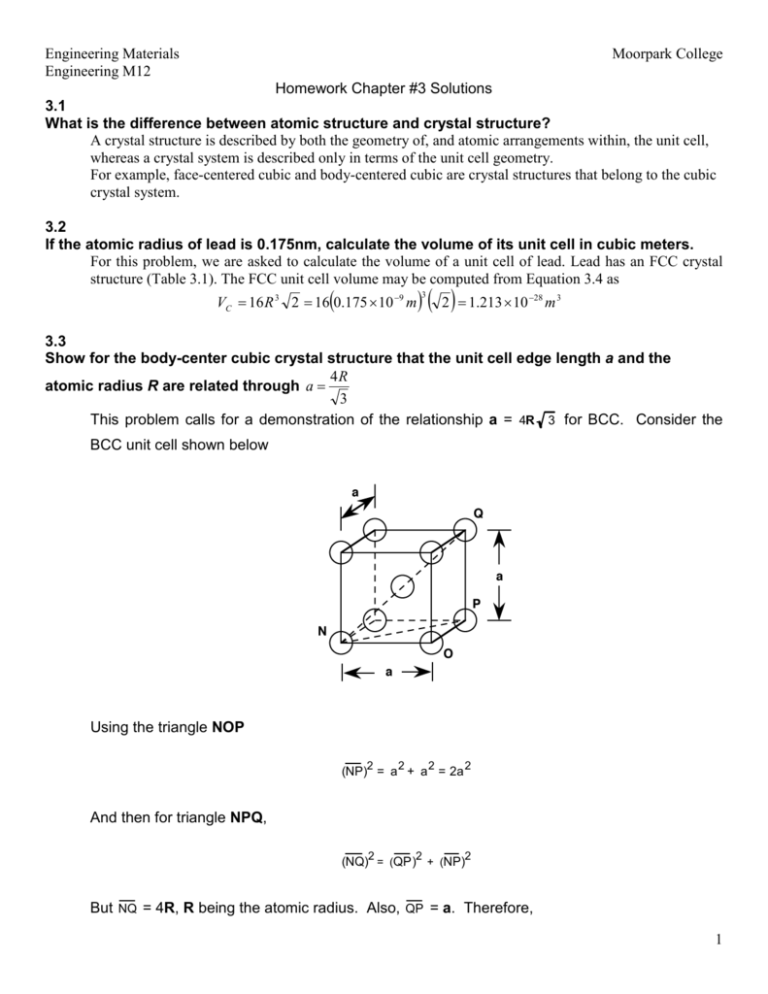
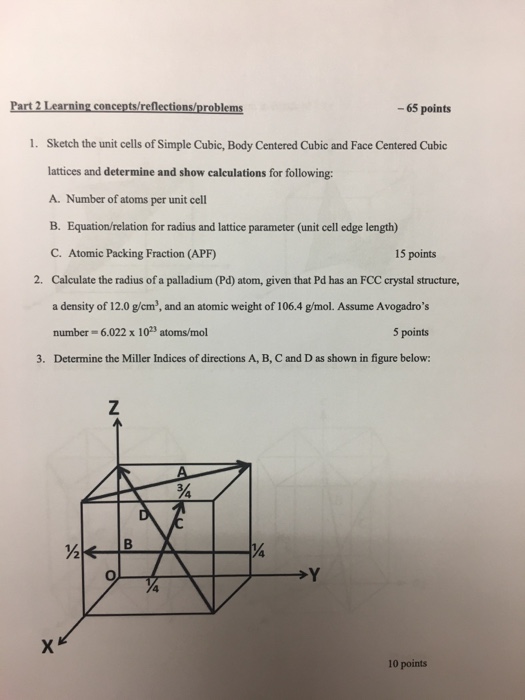
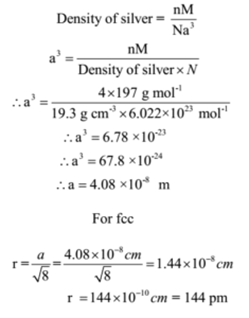
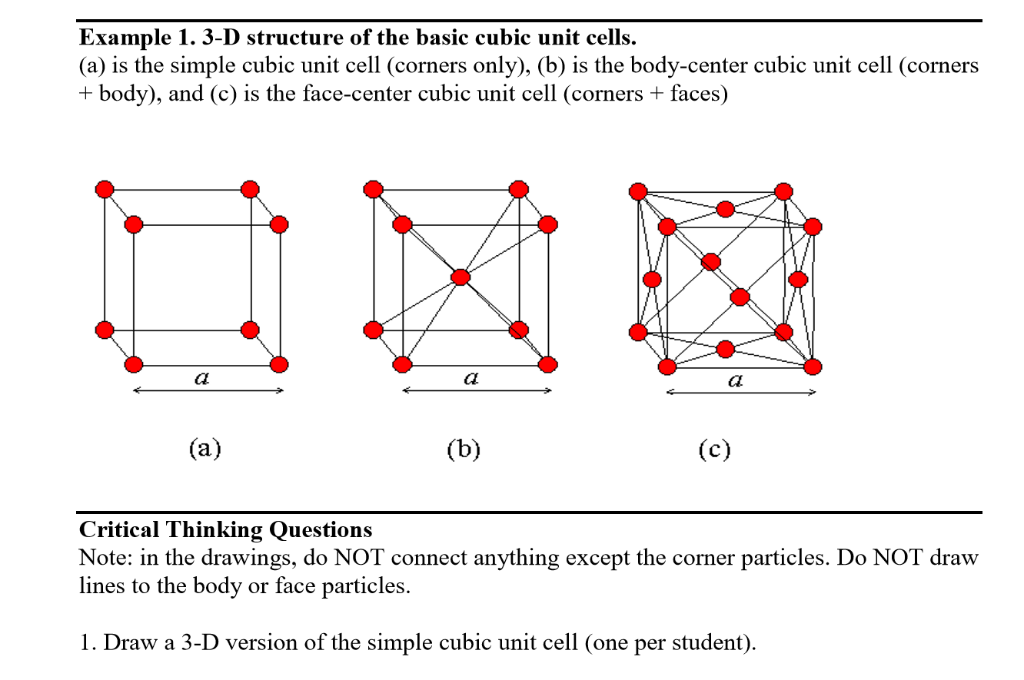
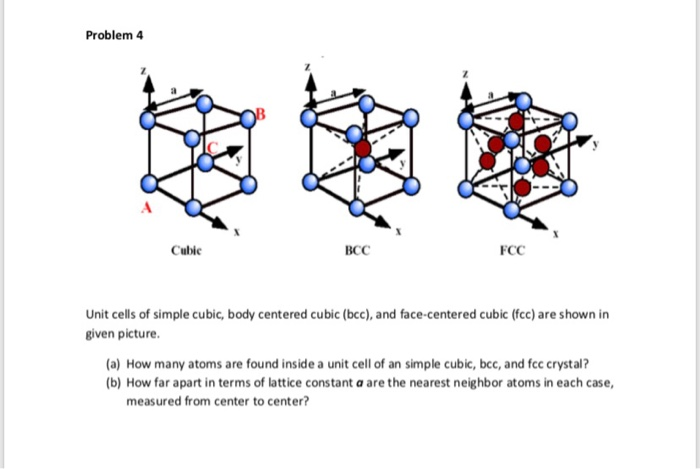
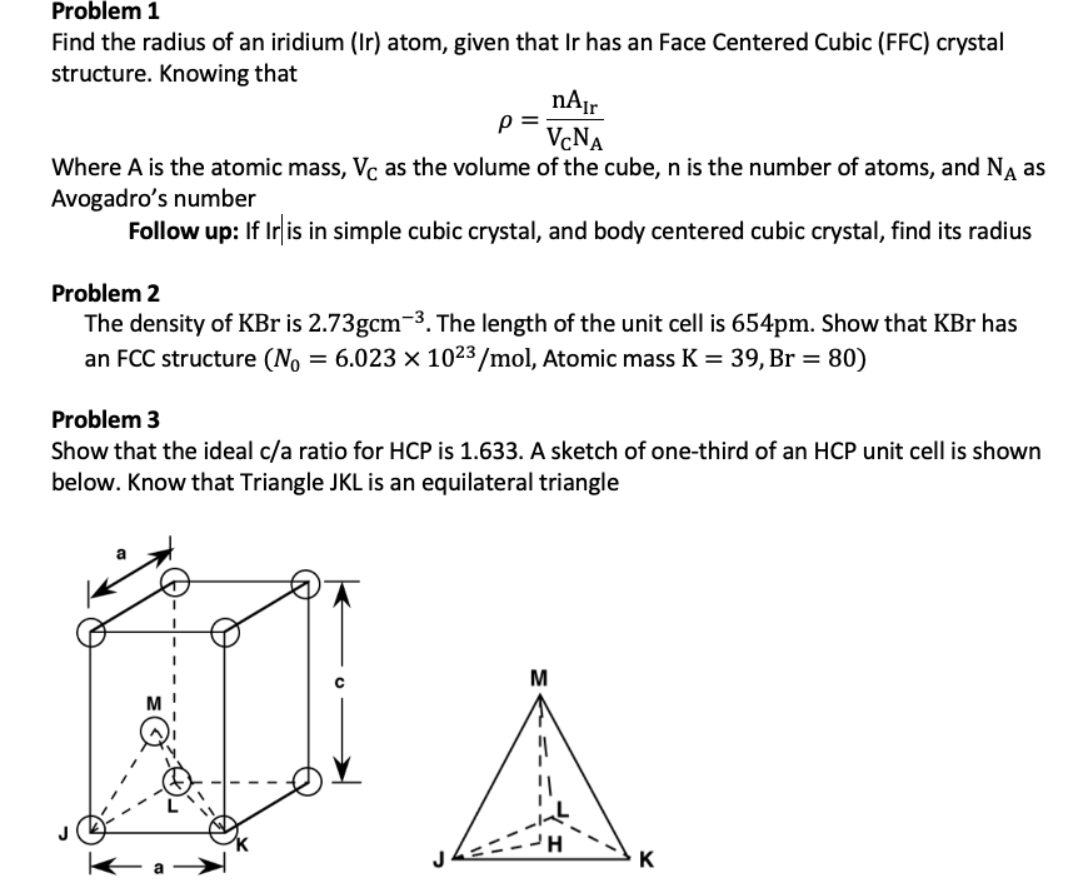
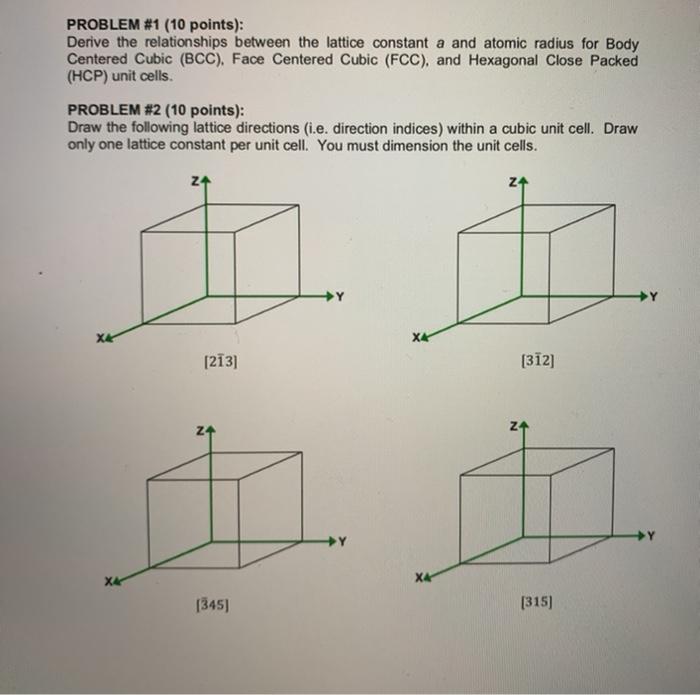
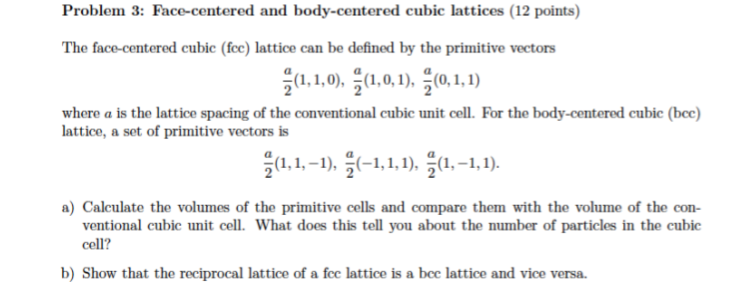
Silver crystallises in a face - centred cubic in cell. The density of Ag is 10.5 g cm^-3 . Calculate the edge length of the unit cell.

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube
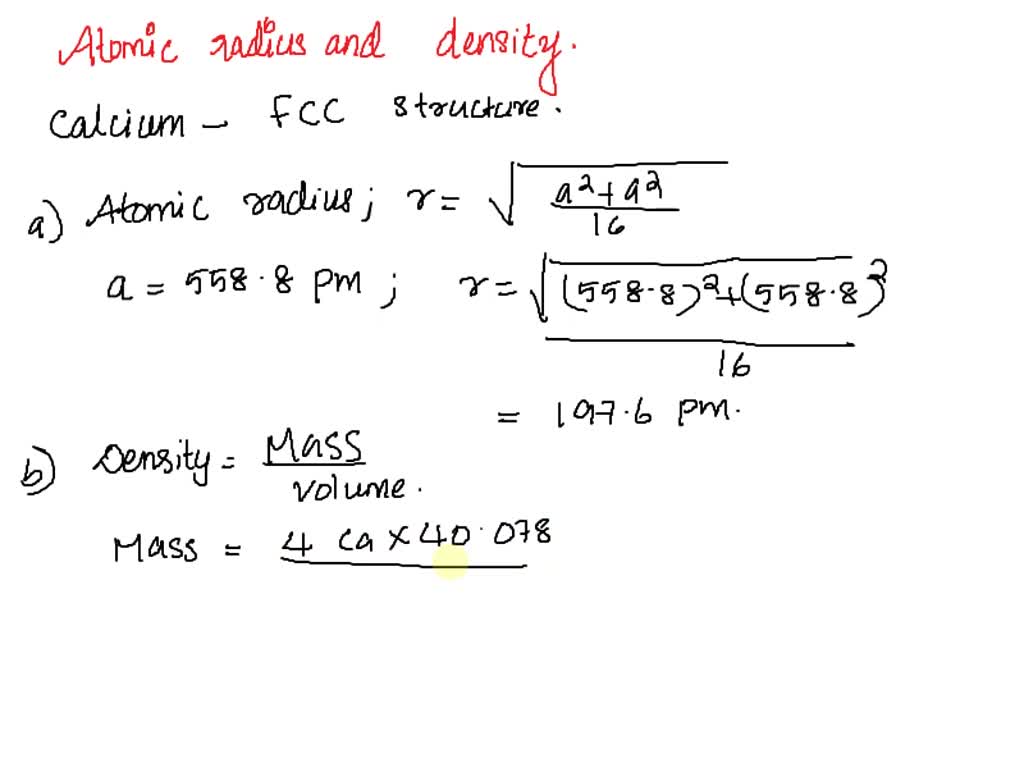
SOLVED: Calcium crystallizes in a face-centered cubic structure. The edge length of its unit cell is 558.8 pm.(a) What is the atomic radius of Ca in this structure?(b) Calculate the density of

Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube

SOLVED: Determine the volume density of the atom in crystals with (a) simple -cubic,(b) body-centered cubic and(c) face-centered cubic crystal structures with a lattice constant a=5A.

HOW TO SOLVE THE EDGE LENGTH OF A FACE-CENTERED CUBIC (FCC) UNIT CELL | WITH PRACTICE PROBLEMS - YouTube

SOLVED: Lead (atomic radius = 175 pm) crystallizes in a face-centered cubic unit cell. Calculate the density of lead given that in face-centered cubic, the edge length of a unit cell =